This page mostly reflects the research carried out in our group until early 2020, before moving and restarting from scratch in Wuppertal.
New topics are being developed and will be showcased more in depth in the near future. Mentioned at times below but not yet reflected in our publications list, some of our current endeavors are to move towards sustainable polymer chemistry (biobased, degradable, recyclable), as well as contribute to novel sensor technologies in collaboration with colleagues of the Wuppertal Center for Smart Materials (CM@S). If you are interested in this too, please contact Guillaume! Most of us having a polymer chemistry background and considering the projects shown below, we are obviously active in the synthesis of novel functional polymers and their modification. We mostly use chain-growth polymerization, particularly reversible-deactivation radical polymerization (RDRP), foremost nitroxide-mediated polymerization (NMP) and reversible addition-fragmentation transfer (RAFT) polymerization, but also copper-mediated RDRP at times. Recently, we established a few routes to access libraries of bifunctional diblock copolymers. Developing novel (functional) monomers for radical (co)polymerization also is a classic endeavor in our group.
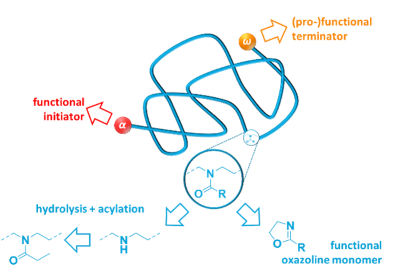
We have also used ring-opening metathesis polymerization (ROMP) and are currently actively working on cationic ring-opening polymerization (cROP) of
2-alkyl-2-oxazolines. See: Polym. Chem. 2020, 11, 593; Eur. Polym. J. 2019, 121, 109281; ACS Macro Lett.
2019, 8, 473; Polym. Chem.
2018, 9, 2679; RSC Adv. 2018, 8, 9471; Polym. Chem. 2017, 8,
1288; Macromol. Rapid Commun. 2017, 38, 1700264; Polym. Chem. 2016, 7, 7488; Chem. Eur. J. 2016,
22, 1511; Acc. Chem. Res. 2015, 48, 1296; Chem. Commun. 2014, 50, 15681; J. Am. Chem. Soc. 2012,
134, 7274; Macromolecules 2012, 45,
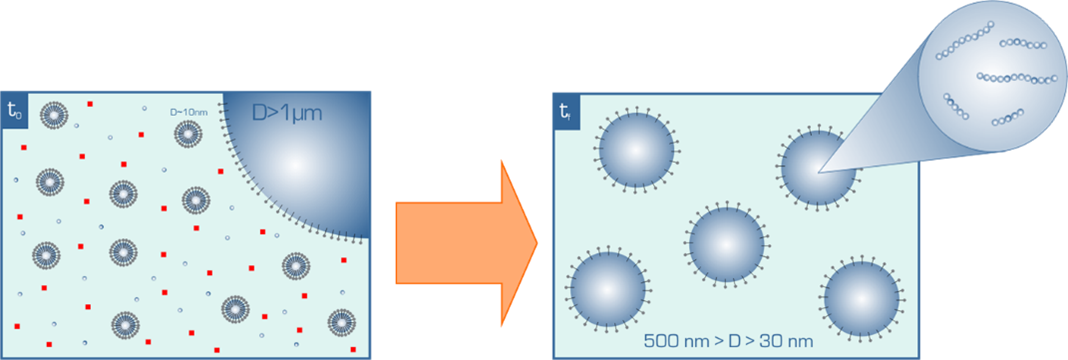
1792; Macromolecules 2011, 44, 462–470. Polymerization in aqueous dispersed media, particularly emulsion polymerization, remains a major technique for the industrial synthesis of polymers as well as for the direct fabrication of polymer particles used in paints, coatings, rubbers, adhesives, binders, etc. We are currently involved in a project with a large company to increase the fraction of biobased and possibly recycled components in technical dispersions. We are open to work on other emulsion-related methods, such as miniemulsion, if this is required.
One of our major contributions in this field concerns the PISA method (polymerization-induced self-assembly), as detailed further below.
See: Polym. Chem. 2021, 12, 50; ChemPhotoChem 2019, 3, 1084; Chem. Commun. 2019, 55, 3741; Angew. Chem. Int. Ed. 2019, 58, 4725; Macromol. Rapid Commun. 2019, 40, 1800551; Biomacromolecules 2017, 18, 2777;
Polym. Chem. 2012, 3,
1526; Macromolecules 2012,
45, 6753; Adv. Polym.
Sci. 2010, 233, 125; Chem. Commun.
2009, 2887; Macromolecules
2008, 41, 2361; Macromol. Rapid Commun. 2007,
28, 1528; Soft Matter 2006,
2, 223; Chem. Commun.
2005, 614. One of our prime research interests lies in the synthesis of nanostructures based on block copolymer self-assemblies. In dispersion See: Small 2018, 14,
1801571; Biomacromolecules 2017,
18, 2777; Macromol. Rapid Commun. 2011, 32, 19. Polymerization-Induced Self-Assembly See: Polym. Chem. 2021, 12, 50; ChemPhotoChem 2019, 3, 1084; Chem. Commun. 2019, 55, 3741; Angew. Chem. Int. Ed. 2019, 58, 4725; Macromol. Rapid Commun. 2019, 40, 1800551; Biomacromolecules 2017, 18, 2777;
Polym. Chem. 2012, 3,
1526; Macromolecules 2012,
45, 6753; Adv. Polym.
Sci. 2010, 233, 125; Chem. Commun.
2009, 2887; Macromolecules
2008, 41, 2361; Macromol. Rapid Commun. 2007,
28, 1528; Soft Matter 2006,
2, 223; Chem. Commun.
2005, 614. In solid state See: Polym. Chem. 2020, 11, 593; Polym. Chem. 2019, 10, 1344; Adv. Funct. Mater. 2018, 28, 1800846; Macromol. Rapid Commun. 2018, 39, 1800231. We aim at developing new methods or improve current methods to impart more stability to proteins, particularly enzymes as it is often desired to employ them in unfavorable conditions for them (temperature, pH, ionic strength). Single-Enzyme Nanogels See: Chem. Sci. 2018, 9, 1006; Small 2016, 12,
1716. Protein-Polymer Conjugates (“Biohybrid Block Copolymers”) See: Angew. Chem. Int. Ed. 2020, 59, 19951; Biomacromolecules 2018, 19, 4250; RSC Adv. 2018, 8, 9471. We currently work with colleagues at the CM@S for the covalent as well as non-covalent functionalization of a range of organic and inorganic materials for sensor applications, particularly using high-frequency technologies. We are also involved in projects in the field of textile functionalization (antimicrobial activity, flame retardancy). In the past, we contributed to the development of several methodologies to tailor the surface chemical functionality of a range of materials, from silicon-based materials to polycarbonate and cellulose. Some are based on simple thermal coupling, while many rely on photochemistry (see further below) Some of these methods should enable control over the spatial distribution of chemical entities and generally proceed in conditions as mild as possible to be applicable to a biological context (see the project about “Three-Dimensional Microscaffolds for Advanced Cell Culture” further below). To this aim, we established photopatterning with various types of reactions: thiol-ene Michael addition, hetero-Diels–Alder cycloaddition, 1,3–dipolar cycloaddition, nitroxide radical coupling, reversible [4+4] cycloaddition, imine or disulfide formation. Besides photopatterning, for some purposes, we use engineering approaches such as microcontact printing or dip-pen nanolithography, the latter in collaboration with Michael Hirtz (INT, KIT). See: Macromol. Rapid Commun.
2020, 41, 2000320; Molecules 2019, 24, 758; Small 2016, 12, 1716; Chem.
Commun. 2017, 53,
1599; Angew. Chem. Int. Ed.
2015, 54, 11388; Adv. Mater. 2015, 27, 2621; Biomacromolecules 2012, 13, 1700; Soft Matter 2012, 8, 7323 In our research mentioned above, we regularly employ photochemical processes. For instance, we more often than not use photoinitiators to start polymerizations or coupling reactions. We also look at photoactivatable functional groups for polymer modification, hydrogel synthesis, and surface functionalization. See: Chem. Commun. 2019,
55, 3741; Polym. Chem. 2019, 10, 1344; ChemPhotoChem 2019, 3, 1084; Small 2018, 14, 1801571; Angew. Chem. Int. Ed. 2017, 56, 15828; Chem. Commun. 2017, 53, 1599; Macromol. Rapid Commun. 2017, 38, 1700264; Adv. Mater. 2016, 29,
1604342; Chem. Eur. J. 2016,
22, 1511; Angew. Chem. Int. Ed. 2016, 55, 3817; Adv.
Mater. 2015, 27, 2621; Adv. Mater. 2013, 25, 6117; Biomacromolecules 2012, 13, 1700; Macromolecules 2012, 45, 1792 Disclaimer: This is a topic which is currently not active but may be an indication of what we can do in terms of 3D printing materials development. See: Angew. Chem. Int. Ed. 2017, 56, 15828; Adv. Mater. 2016, 29,
1604342; Angew. Chem. Int. Ed. 2016, 55, 3817; Adv. Mater. 2013, 25,
6117.
Polymer Synthesis and Modification
Polymerization in Dispersed Media
Self-Assembled Block Copolymer Nanostructured Materials
We are particularly focusing on reactive nanostructures which can be obtained by
self-assembly either in solution or in solid state. The resulting materials may find applications in domains such as catalysis, biosensor design, medicine, or biology, to cite but a few.
We synthesize nanoparticles based on the self-assembly of block copolymers in a liquid phase or more precisely as dispersions. After synthesis of an amphiphilic block copolymer precursor, core-shell nanoparticles can indeed be obtained by nanoprecipitation. One of our original targets in this field was to establish versatile platforms for enzyme immobilization,
ideally in a specific/controlled manner. This is also relevant to the field of targeted drug delivery, bionanoimaging, or simply nanoparticle stabilization.
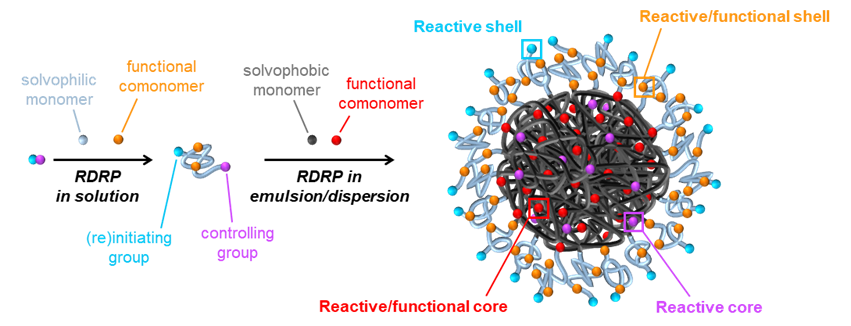
Besides the classic nanoprecipitation method mentioned above, we use the technique of polymerization-induced self-assembly, so-called PISA, as an efficient method based on emulsion or dispersion polymerization, giving access to core-shell nanoobjects in a reduced number of synthetic steps and higher concentrations. The PISA method was first developed within the PhD thesis of Guillaume in the group of Prof. B. Charleux, back in 2004-2005. It has now been widely adopted in the synthetic polymer community, particularly by those working with radical polymerization, and popularized by the groups of Prof. S. P. Armes, who unraveled many of the subtleties of the process.
We are particularly interested to use the PISA method for new applications and are also investigating some fundamental aspects governing the formation of various nanostructures such as spheres, worm-like micelles/fibers, and vesicles. For this, we look at alternative polymerization methods (e.g., ROMP) and stabilizers (e.g., polyoxazolines).
One of our aims is to develop versatile polymer films which can template the ordering of biomolecules at a scale approaching the dimensions of the biomolecules themselves. We are currently looking for simple systems which allow maximal flexibility in terms of functionality. For this purpose,
we synthesize functional block copolymers by reversible-deactivation radical polymerization techniques and study their self-assembly.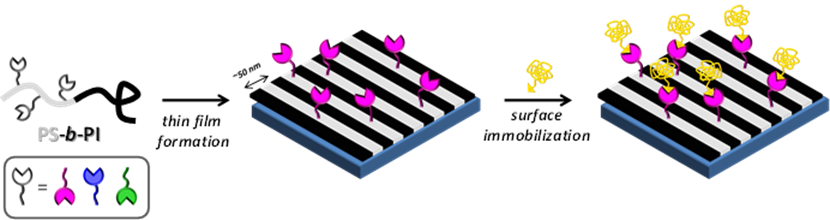
Protein Stabilization
One very efficient method to increase the range of conditions under which enzymes can be employed is their encapsulation in a thin layer of hydrogel at the single-molecule level. The advantage of the so-called single-enzyme nanogels (SENs) resides therefore in a minimal change in the biocatalyst dimensions, which may be very crucial in some applications. For instance, in comparison to classic encapsulation in larger micro-/nanogels, diffusion processes may be less hindered. During surface immobilization, the density of biocatalysts may also be significantly higher.
We have for instance demonstrated the possibility of grafting as-formed SENs onto surfaces by thio-Michael addition, in very mild conditions. It allowed to employ microcontact printing for the establishment of stable protein patterns.
Besides surface immobilization or crosslinking, enzymes – as well as proteins in general – are often stabilized by attachment of polymer chains through their end group to the surface of the biomolecule. We are looking at novel chemical routes for this purpose, particularly those which enable straightforward variation of the polymer structure. In this regard, the most largely employed polymer for this purpose is polyethylene glycol (PEG), as seen for instance in some commercial drugs. However, for the future it is important to look for alternatives in order to offer more flexibility and counter possible issues linked to the immunogenicity of PEG or provide further enhanced characteristics. 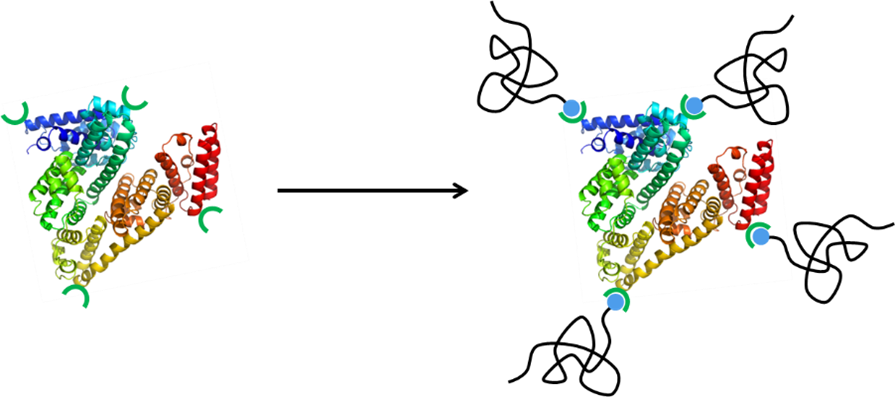
Surface Functionalization
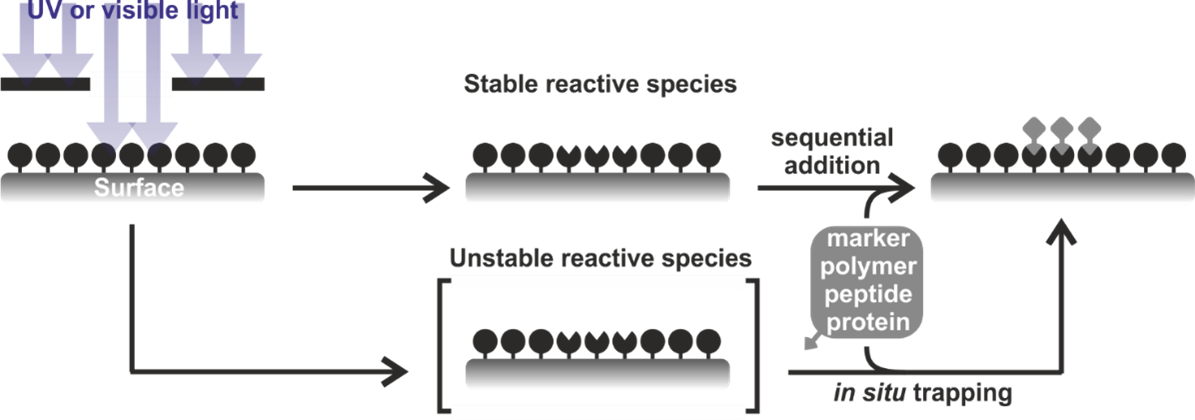
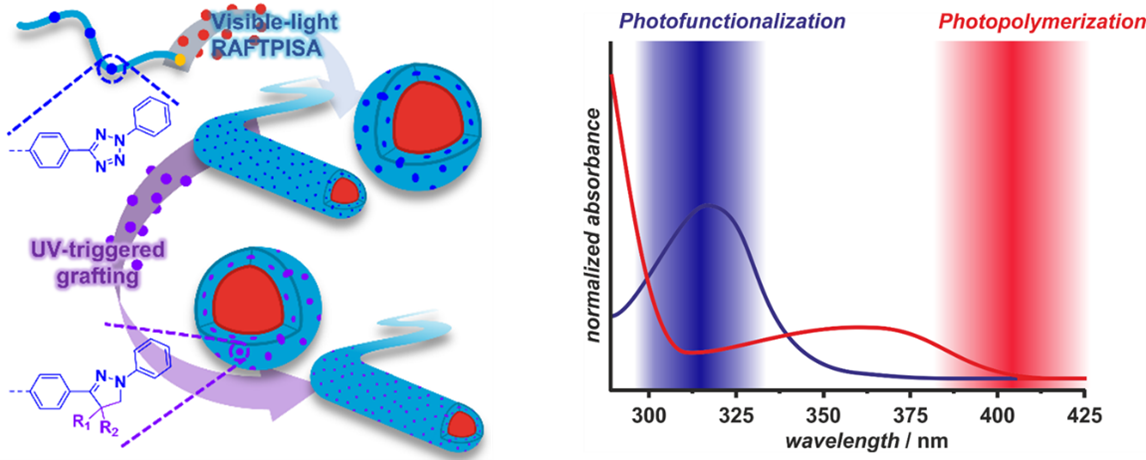
Polymer Photochemistry
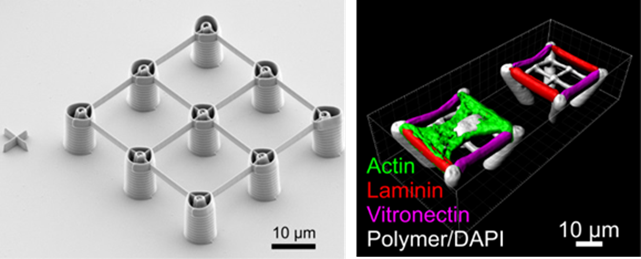
Three-Dimensional Microscaffolds for Advanced Cell Culture
Until recently, most studies on cell behavior were carried out on flat surfaces, typically in Petri dishes. However, in natural environments, cells are not only organized in a two-dimensional fashion (e.g., skin cells) but also in three dimensions. Several studies showed that the three-dimensional geometry of cell culture scaffolds is essential for cells typically growing in such conditions. Furthermore, mechanical properties of the material as well as the surface chemistry play crucial roles. Therefore, in a project with the teams of Christopher Barner-Kowollik, Martin Bastmeyer, and Martin Wegener (all at KIT), we developed methods to produce 3D free-standing microscaffolds with spatially tunable surface functionality. For this purpose, a very fine 3D printing technology named direct laser writing (DLW) was employed together with custom polymerizable inks. These incorporate photoreactive groups that are able to survive the photochemical DLW process. This led to photoreactive microscaffolds, which can subsequently be surface-functionalized using custom-made DLW setups.